Will New Anti-COVID Pills Live Up to the Hype?
Since the onset of the COVID-19 pandemic in 2020, researchers have been continuously striving towards developing effective therapies to contain the virus and minimize its implications on the affected individuals. While wearing masks, social-distancing, sanitization, and vaccination remained the leading options for preventing the transmission of infections, the upsurge in the latest coronavirus mutation, Omicron variant, has created an urgent necessity for antiviral therapies that reduce the risk of coronavirus progression.
The United States Food and Drug Administration (USFDA) has granted authorization of two COVID-19 pills, NIRMATRELVIR (branded as PAXLOVID) by Pfizer and MOLNUPIRAVIR by Merck and Ridgeback Biotherapeutics for high-risk people. Both the pills have the ability to reduce the viral load by more than tenfold and significantly reduce contagiousness to others.
The new antiviral pills are designed to work against the coronavirus and its variants, reducing the need for hospitalizations and mortality rates. Currently, health authorities are providing antiviral pills to individuals with mild or moderate COVID or who have a high risk of serious illness due to chronic issues like diabetes, cardiovascular disorders, compromised immune functioning, etc.
The antiviral pills are required to be taken home as soon as possible, within five days of the start of the symptoms. Fever, cold, headache, loss of taste or smell, muscle and body aches are some of the common symptoms that people are experiencing with the new coronavirus variant. Starting the pills in a short window is the real challenge due to delay in testing and getting a prescription.
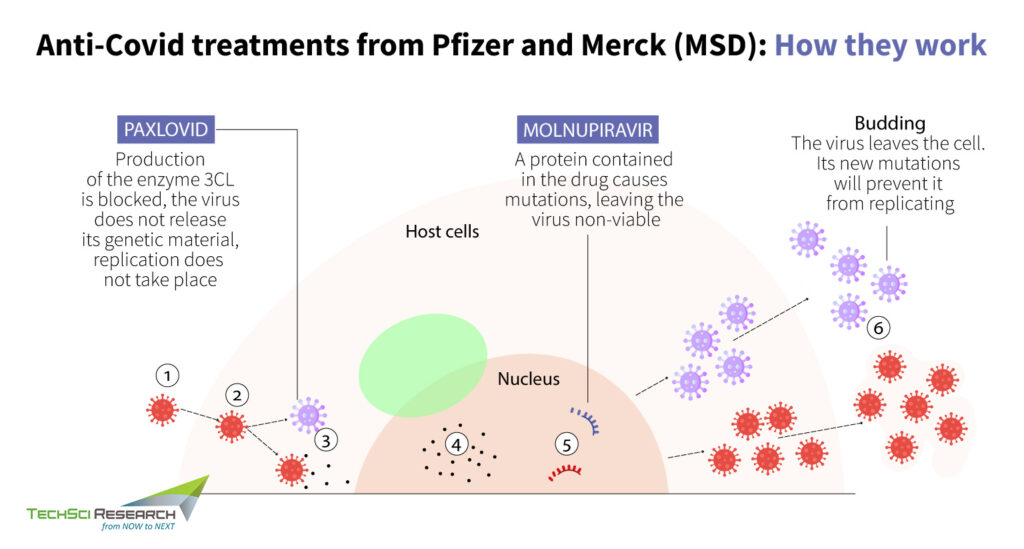
Mechanism of Paxlovid Against COVID-19 Infection
Paxloid drug is designed to slow the spread of infection by disrupting specific processes in the viral assembly, choking the virus’s ability to replicate and cause damage. The antiviral medication has a cellular component called a protease that cuts virus’s polyprotein, a large, clumped mass generated by replicating the action of coronavirus for survival, into small and workable pieces. Paxlovid is a combination of two antiviral drugs, nirmatrelvir, a novel main protease inhibitor that cuts viral products into smaller pieces, and ritonavir, which enables nirmatrelvir to remain active in the body for more extended periods at higher concentrations.
Thus, the anti-COVID pills can be considered a huge advancement for all kinds of coronaviruses since it reduces the chances of the virus developing resistance. In clinical trials, Paxlovid showed an 89% reduction in hospitalization or death in those who received treatment within just three days of symptom onset. Hence, the authorization of Paxlovid prevents disease progression and reduces the burden on hospitals. Since nirmatrelvir has previously shown efficacy against variants of concerns like alpha, beta, gamma, lambda, and mu, it has the potential to maintain robust antiviral action against omicron.
Some of the side-effects of taking the treatment are altered sense of taste, diarrhea, high blood pressure and muscle aches. Individuals who have any allergies, liver or kidney disease, pregnant or breastfeeding a child or any serious illnesses must refrain from taking the medication.
Mechanism of Molnupiravir Against COVID-19 Infection
Molnupiravir is a polymerase inhibitor that increases the frequency of viral RNA mutations and impairs coronavirus replication. SARS-CoV-1 and SARS-CoV-2 use an RNA-dependent RNA polymerase (RdRp), a cellular component that works like a photocopying machine for the reproduction and transcription of their RNA genome. Most antiviral drugs can target viral polymerase to terminate RNA chain elongation of the virus, but SARS-CoV-2 carries an exonucleolytic that remove misincorporated nucleotides.
However, Molnupiravir is turning out to be a promising treatment since it can target the RdRp of SARS-CoV-2 like the previously approved drug, Remdesivir. The oral medication acts as a mutageninzing agent that inserts itself into the viral instructions, which RdRp is copying to proliferate. Thus, causing an ‘error catastrophe’ during viral replication, Molnupiravir inhibits the virus from replicating further. Molnupiravir can trigger mutations in other RNA viruses and thus treat a range of viral diseases. Earlier the oral medication was used to treat influenza and now in clinical studies, it is found to be highly effective against SARS-CoV-2.
The anti-viral COVID pills is highly effective in reducing the incidence of hospitalization or deaths related to infection. Some of the side effects of Molnupiravir include nausea, dizziness, diarrhea, and headache. Individuals less than 18 years or hospitalized patients with severe symptoms should not take Molnupiravir. Molnupravir’s mechanism of action against COVID-19 could induce viral mutations, which could form mutations within human DNA, whereas Paxloid has shown no signs of mutagenic DNA interactions. Also, these anti-COVID pills are substitutes for the vaccines; thus, one must take proper measures and get vaccinated to prevent infection.
How Anti-Covid Pills Differ from Vaccines?
Unlike vaccines, the antiviral pills are not a preventive strategy. Rather, they treat an infected person with the symptoms arising from the viral infection. Vaccines stimulate the production of antibodies to strengthen the body’s defense system against the infection. To do so, vaccines put the immune system through a ‘’rehearsal’’ mode before it has to really fight off the real coronavirus. In our bodies, DNA is the nucleus that instructs the rest of the cells what proteins to build via a molecule called messenger RNA (mRNA).
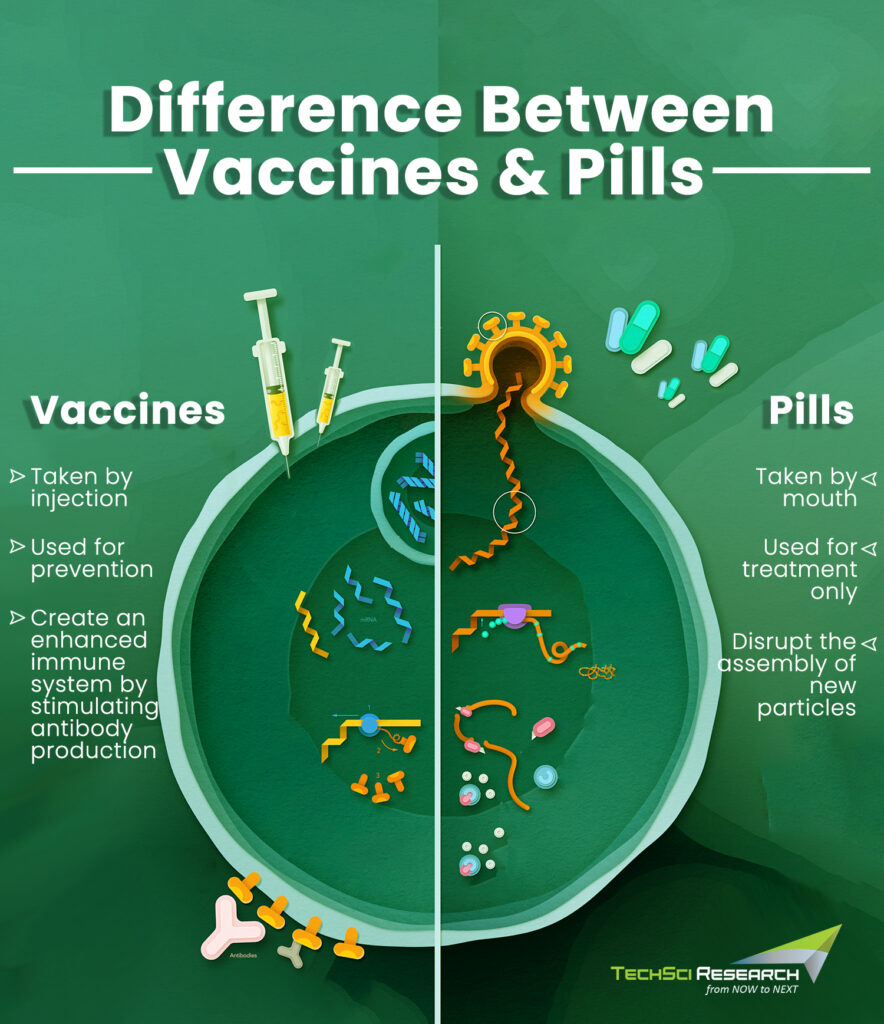
Thus, mRNA vaccines inject antigen-building instructions to start a complex assembly line using our own cellular machinery to build spike proteins from scratch, which disintegrates a few days once the immune system produces antibodies. Further, the antibodies wait for an opportunity to attack the real spike protein that might enter the body. However, these antibodies decrease over time and thus booster vaccines are required to maintain a long and strong line of defense against the pathogen.
Pharmaceutical companies are racing to find new life-saving therapies for the treatment of Covid to break the chain of virus transmission. Two of the drug treatments highly recommended by the World Health Organization for Covid-19 are as follows.
Baricitinib
Hospitalized patients with coronavirus infections tend to develop an intense hyperinflammatory state, which could result in damage to several organs. Baricitinib oral drug treatment is highly effective for patients with critical COVID symptoms. The medication belongs to the class of drugs called Janus kinase (JAK) inhibitors that are widely used for the treatment of rheumatoid arthritis. Given with corticosteroids, Baricitinib could potentially reduce the inflammation and associated immunopathologies observed with COVID-19 patients.
The medication helped combat the overstimulation of the immune system known as cytokine storm, which can result in fatal outcomes in critically ill COVID-19 patients. In clinical studies, it was found that baricitinib significantly improved the survival rate and reduced the need for ventilation. Besides, baricitinib reduces the length of hospital stay. The drug is not recommended for patients with mild COVID symptoms and with extreme caution since the drug has the potential to harm and limited accessibility.
Sotrovimab
The World Health Organization recommends the use of Sotrovimab, a monoclonal antibody for non-severe COVID-19 patients. The drug created by GlaxoSmithKline in collaboration with Vir Biotechnology received emergency clearance in European Union in December 2021. The drug should be administered to patients with non-severe COVID-19 symptoms who are at high risk of hospital admission. Patients with a compromised immune system, chronic symptoms like diabetes, hypertension, obesity, or unvaccinated elder individuals make the ideal candidate for this drug treatment. Those affected with Delta or Omicron variant can also find relief from the use of this medication.
REGEN-COV (Casirivimab and Imdevimab)
The US Food and Drug Administration (FDA) authorized the emergency use of REGEN-COV for use in patients who are hospitalized, require oxygen therapy, or mechanical ventilation due to Covid-19. Monoclonal antibodies, casirivimab and imdevimab are recombinant human mAbs that bind to non-overlapping epitopes of the spike protein of SARS-CoV-2. The investigational medicine is authorized for use in only adolescents and adults who weigh more than 40 kg or 88 pounds. Some of the possible side-effects of REGEN-COV include allergic reaction, fever, chills, nausea, headache, chest pain, muscle aches, feeling faint, dizziness, sweating, etc. The medication could also reduce your body’s ability to fight against the virus or build an immune response to the vaccine.
Conclusion
While vaccines have proven to be highly effective in reducing the mortality rate across the world, vaccine coverage remains insufficient. Thus, it is safe to say that antiviral medications are going to become the mainstream treatment option with their wider accessibility and a growing number of options. Combination of anti-COVID pills and vaccines can prove to be a powerful tool for controlling the outbreak. Besides, other treatment alternatives for SARS-CoV-2 like monoclonal antibody therapies from Eli Lilly, Regeneron and GlaxoSmithKline have been granted authorization for their commercial use for COVID-19 treatment, which will further help to contain the transmission of the virus and save lives.



