CAR T-Cell Therapy: Expanding Horizon of Oncology Treatment
Cancer is one of the leading causes of death worldwide. In 2020, around 10 million people lost their lives to cancer, and the number is expected to rise to 29.5 million by 2040. Over the years, many conventional treatment approaches have been developed for cancer like chemotherapy, bone marrow transplant, radiation therapy, surgery, and more such interventions. However, their limited effectiveness with the heterogeneity of cancer cells has led to a more improved therapeutic approach that enhances the patient’s immune system to attack cancer cells without causing major side effects. Chimeric Antigen Receptor-T cell (CAR-T) therapy has emerged as one of the most promising treatments for cancer patients, which has shown effective and durable clinical responses in recent years. The cell gene therapies support the patient’s immune system and facilitate the elimination of cancer cells from the body.
How does CAR T-cell Therapy Work?
CAR-T cell gene therapy is a kind of immunotherapy that involves genetically engineered T-cells to strengthen the immune system for fighting against diseases. T-cells are either taken from patients or donors and are then modified in a laboratory to provide them the power to recognize and kill cancer cells. When T-cells are infused into the patient, the cells multiply and stay in the body as “living drugs,” which multiply into hundreds of millions in the patient’s body to target the cancer cells that harbor the foreign antigen on their surfaces. CAR T-cells may help eradicate all the cancer cells and live in the body for months after infusion. This immunotherapy treatment is used for treating acute lymphocytic leukemia (ALL) in kids and young adults. Many clinical trials are underway to determine its efficacy against different kinds of cancers.
CAR T-cell therapy leverages the natural ability of the body to target and destroy malignant cells. CARs are genetically engineered surface receptors designed to recognize tumor cells as dangerous and bind to antigens found on them. When the extracellular domain binds to a tumor antigen, the CAR is activated, which generates a cytotoxic response and destroys the tumor cells. The CAR extracellular domain consists of tumor-specific monoclonal antibodies, and the intracellular portion consists of the signaling portion of the receptor. Full activation of endogenous T cells requires two signals, one from intracellular signaling and the second from a co-stimulatory domain, and then the CARs work to replicate both. Then, the chimeric CAR molecule integrates the specificity of a monoclonal antibody with the cytotoxic and memory capabilities of endogenous T cells to destroy the cancer cells into the patient’s body.
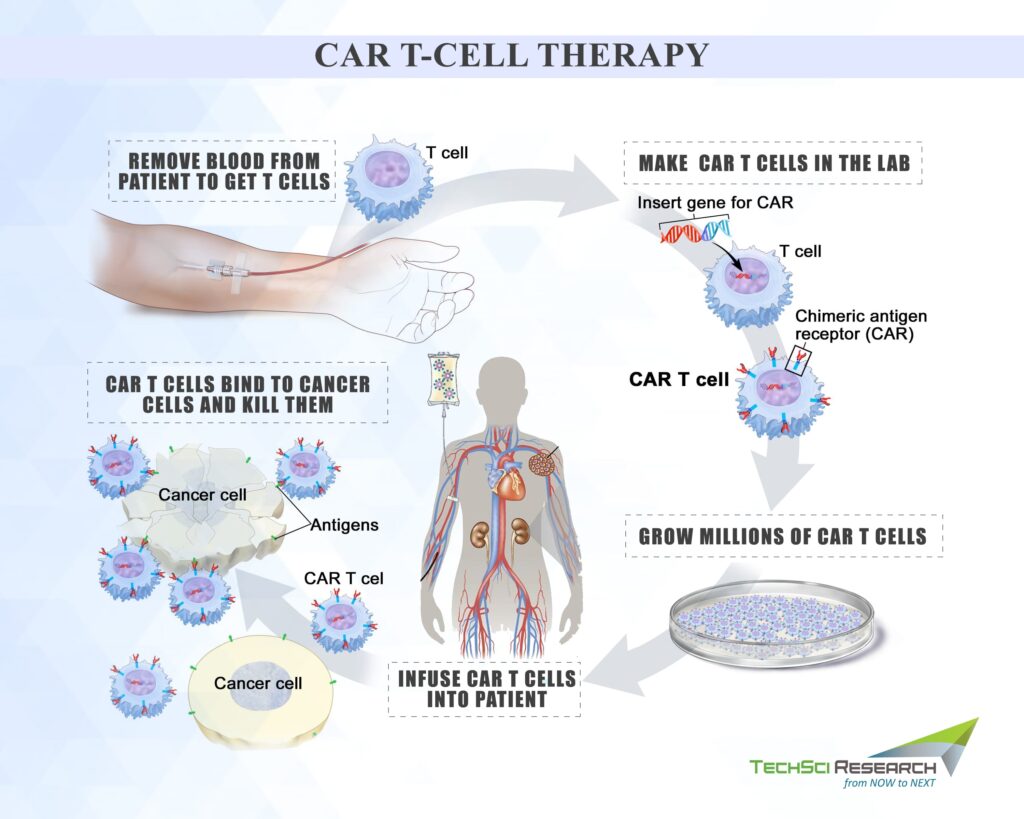
Manufacturing of CAR T-cell therapy
CAR T cell manufacturing requires a high degree of consultation from the primary oncology team, which determines the patient’s eligibility for CAR T-cell therapy and provides continuing care throughout the process. Although protocols for each product vary, the CAR T-cell therapy mainly involves the following steps:
Patient’s Evaluation
Generally, patients with an adequate number of T-cells for collection and tumors positive for CAR target are found eligible for CAR T-cell therapy. The patients should not have an active infection, and the organs must have an adequate performance status. Patients with cardiovascular, immune, or neurological disorders are found to be ineligible for the treatment. Hence, pre-screen testing is mandatory to check the eligibility criteria of the patients and manufacture CAR T-cell products.
Leukapheresis
The patient’s treatment is frequently altered to increase the concentration of white blood cells in the body, which can be collected for further interventions. The healthcare provider might ask the patient to avoid corticosteroids or salvage chemotherapy for a certain period. Once the patient has an adequate number of white blood cells, he/she undergoes a leukapheresis procedure, which involves passing the blood through a machine that takes out white blood cells and returns the red blood cells and plasma into the bloodstream. The collected cells may be frozen and sent to the highly specialized manufacturing facilities, depending on the product. Highly skilled and knowledgeable staff enrich the lymphocytes and modified with cell subsets such as CD4+ and CD8+ T cells.
Activation of T-cells
The isolated T cells are placed in culture and exposed to antibody-coated beads to activate them. The capability of T cells to expand in vitro can vary from patient to patient. Viral vectors are used to introduce the CAR gene into T cells for permanent genome modification and CAR expression. The developer can also use transposon systems or direct mRNA transduction to insert the CAR gene into DNA cells. For generating enough CAR T cells for therapy, cells are expanded using a variety of culture systems. The scientists then amplify the engineered cells in a bioreactor, and the physician inserts it into the patient.
Pre-conditioning Therapy
The patient has to undergo chemotherapy before the infusion of CAR T-cells to decrease endogenous lymphocytes. The depletion of white blood cells in the body releases endogenous intracellular inflammatory cytokines, promoting CAR T-cell activity and reducing immunosuppressive cells that may threaten their expansion.
Infusion of CAR T-cells into the Patient
The genetically modified cells are delivered to the infusion site into the patient in the inpatient setting or in the outpatient setting with careful monitoring.
The manufacturing process of CAR T-cells is a complex procedure and creates unique challenges since they are not typical drugs. CAR T-cells are living cells, and thus manufacturing has to depend on the physiological workings of the cells to express the CAR gene in appropriate quantity. Besides, the manufacturer cannot regulate the oncogene expression and thereby promote tumor growth. Moreover, it can be challenging for the CAR gene to integrate into the T cell’s genome, which can impact the safety as well as the function of the cell.
CAR T-cell therapy has garnered interest from patients, families, and healthcare providers to treat hematologic malignancies. Most advanced clinical trials have evaluated CD-19 directed CAR T-cell therapy, but the investigative pipeline of CAR T-cell continues to promise major expansions. Currently, ten cells including CD19, CD20, GD2, CD30, CD33, HER1, Meso, and EGFRVIII are being deployed due to their promising 90% remission rates. Pressing medical needs for cancer therapies are pushing regulatory agencies to expedite the approval process with more funding and guidance for research. More than 500 CAR T-cell therapies are under trial, including four products, Kymriah, Yescarta, Tecartus, and Breyanzi, which are already being used for cancer treatment.
Growing Commercial Application of CAR T-cell Therapies
Kite Pharma’s Yescarta was the first CAR T-cell therapy to be approved by the US Food and Drug Administration (FDA) for the treatment of adult patients with relapsed or refractory large B-cell lymphoma. Yescarta is a CD19-directed genetically modified autologous T-cell immunotherapy made from patients’ own white blood cells. Yescarta CAR T-cell therapy is different from other cancer treatments, and it is used when other kinds of treatment interventions have failed to mitigate cancer. The Japan-based pharmaceutical company Daiichi Sankyo has collaborated with Kite to expand the usage of Yescarta in Japan. The country has the second-largest number of people diagnosed with non-Hodgkin lymphoma globally.
In December 2021, Novartis announced the next generation CAR-T platform, T-Charge, to revolutionize CAR T-cell therapy. T-Charge will serve as the foundation for various investigational CAR T-cell therapies and enhance its therapeutic potential. The early clinical data from first-in-human dose-escalation trials showed promising results for improving existing CAR T-cell therapies as the platform could provide the ability to self-renew and mature. T-charge eliminates the need for an extended culture time outside the body as it enables expansion inside the patient’s body. Application of T-Charge could offer patients the likelihood of better and more durable responses while making the implementation process efficient with streamlined quality control. In October 2020, US FDA granted approval for Tecartus, the first and only approved chimeric antigen receptor (CAR) T cell therapy for treating adult patients suffering from relapsed or refractory mantle cell lymphoma (MCL).
Future of CAR T-Cell Therapy
Currently, only CAR T-cell therapies targeting CD-19 biomarkers are used for cancer treatment. Lack of CD-19 on cancer cells can even lead to relapse. Thus, expanding the scope of target molecules can be a potential gamechanger for cell therapy as it would become possible to target multiple antigen receptors at the same time. At present, B-cell maturation antigen (BCMA) for myeloma is a cell surface receptor excessively produced in multiple myeloma.
Another biggest challenge faced by CAR T-cell therapy is the cytokine release syndrome and other neurological complications. However, scientists worldwide are developing technologies and finding innovative ways to reduce the severity of CRS and other modifications. Some measures to combat the CRS barriers are early rise in biomarkers, prophylactic interventions, and dose adjustments.
The allogeneic CAR T-cell therapies are being explored to overcome the limitations associated with autologous therapies, such as extensive manufacturing time and effort for customized treatments particular to the patients. On the contrary, allogeneic therapies do not have any complications and are similar to the organ transplant process. In any case, the CAR T-cell therapies will continue to change the paradigm of oncology treatment and improve healthcare.
According to TechSci Research report, “Global CAR-T Cell Therapy Market, By Product Type (Yescarta, Kymriah (Tisagenlecleucel), Tecartus (brexucabtagene autoleucel), Breyanzi (lisocabtagene maraleucel), Abecma (idecabtagene vicleucel), Others), By Tumor Type (Hematological Malignancies, Solid Tumors), By Indication (DLBCL, ALL, FL, MCL, Others), By Treatment Type (Single Treatment, Combination Treatment), By Targeted Antigen (CD 19, BCMA (B-Cell Maturation Antigen), Others), By End User (Hospitals, Speciality Clinics, Ambulatory Surgical Centers, Others), By Region, Competition Forecast & Opportunities, 2026”, the global CAR T-cell therapy is anticipated to reach USD6.13 billion by 2026, owing to factors such as rising prevalence of cancer and growing need for therapeutic solutions. Rise in clinical trials due to huge investments and R&D initiatives is propelling the growth of the global CAR T-cell therapy market.