Xenotransplantation: The Future of Organ Transplantation
The increasing life expectancy of humans has led to a rising number of patients living with chronic disorders, which may ultimately require organ transplantation for the treatment of their condition and prevent end-stage organ failure. Although clinical transplantation is an effective solution to save lives, the imbalance between organ supply and demand for human organs remains a challenge for the medical community. According to organ donation statistics, 17 people die each day waiting for an organ transplant. Hence, xenotransplantation, or cross-species transplantation, is considered a promising alternative to resort to the shortage of human tissues, prohibiting most patients from undergoing transplants. The possibility of transplanting animal cells, tissues, or organs in humans could improve mortality rates for conditions such as chronic obstructive pulmonary disorders, diabetes, liver cirrhosis, polycystic kidney diseases, etc.
Domestic Pigs: Optimum Organ Donor
Domestic pigs are considered the best donors of biological materials for xenotransplantation due to their anatomical and physiological similarities to humans, low risk of xenozoonosis, short maturation period, and large litter size. One major obstacle with xenotransplantation is transplant failure, as the body mounts an immune response against the xenografts (transplanted organ or tissue) and rejects it, which could result in the recipient’s death.
Hence, the genes of domestic pigs are modified to prevent the recognition of their organs by the human recipient’s immune system and inhibit the process leading to xenograft rejection. Genetically engineered proteins express the human CD47 protein that reduces the risk of organ rejection, and the immune-suppressing drugs target coagulation and inflammation, which increase the duration of organ function. Several techniques that enable precise genetic modification of animals include pronuclear or cytoplasmic microinjection, somatic cell nuclear transfer, viral transduction of DNA.
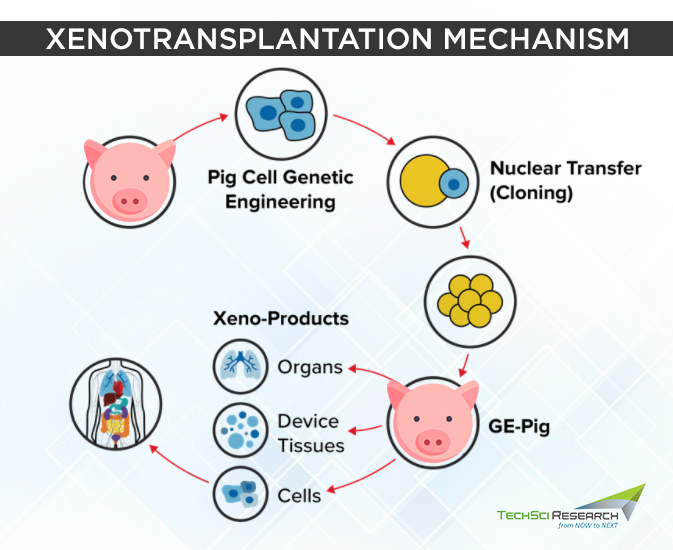
In September 2021, a team of surgeons at New York Langone Medical Center successfully transplanted a genetically engineered pig kidney into a deceased donor kept on a ventilator. The xenograft led to the production of urine for 54 hours without showing any signs of organ rejection.
Urine production and creatinine levels were completely normal and equivalent to what is expected from a human kidney transplant. Alpha-gal, the gene responsible for a rapid antibody-mediated rejection of porcine organs by humans, had been knocked out in the donor pig. Additionally, the pig’s thymus gland also educated the immune system to stave off novel immune responses.
In January 2022, surgeons at the University of Maryland Medical Center conducted a successful transplant of genetically modified pig heart into a 57-year-old with terminal heart disease. The organ transplant is a medical breakthrough as the pig heart continues to function as a human heart without immediate rejection by the body. The patient had been on heart support for around two months, and the untreated high blood pressure and other health problems made him ineligible for a human heart transplant.
The heart was availed from a Virginia-based company, Revivicor, that had been engineering pig organs for nearly two decades. The pig heart had ten hefty genetic edits, out of which three of them wiped out sugar molecules that stimulate an immune response, six bolster the chance of the human host accepting the heart, and the last edit restricts the pig heart’s size.
Once removed from the pig, the heart is bathed in hormones, nutrients, and cocaine. Even the patient was kept on heavy doses of antibody-drug anti-CD40 to dampen his immune system and inhibit communication between different immune cells. In the end, the transplant surgery turned out to be successful; however, the patient is still under observation.
Pig liver xenotransplantation is a more challenging procedure compared to transplanting the heart and kidney due to more complex molecular structures in the liver xenografts. Severe thrombocytopenia can occur after pig liver xenotransplantation, leading to lethal hemorrhage. Besides, pulmonary xenografts release large quantities of vWF (a type of glycoprotein) than heart and kidney xenografts, which can exacerbate even more coagulation dysfunction.
Clustered Regularly Interspaced Short Palindromic Repeats-cas9 (CRISPR/ cas9)
Clustered regularly interspaced short palindromic repeats-cas9 (CRISPR/ cas9) has proved to be a viable gene-editing tool to produce genetically modified pigs easily and at a rapid rate. The technology has revolutionized many aspects of genetic engineering research by offering greater precision and providing acquired ‘immunity’ mechanisms against viruses.
Recognizing the target DNA on RNA-dependent molecular scissors, CRISPR adds versatility to genome engineering and allows editing of different cell types to generate transgenic animals in a short amount of time. CRISPR technology is also used in a wide variety of medical fields such as oncology, neuroscience, or developmental biology due to its scalability and multiplex targeting. Additionally, the application of CRISPR is able to eliminate porcine endogenous retroviral (PERV) sequences from the genome of porcine cells.
Cell Xenotransplantation
Patients suffering from degenerative and auto-immune disorders could benefit from cell replacement therapies designed to provide a long-lasting restoration or amelioration of disrupted cellular functions. Besides, cell replacement therapies for replacing cell types such as hepatocytes, bone marrow, or umbilical cord stem cells are utilized to prevent severe conditions.
However, the lack of human donors is one of the major obstacles standing in the way of the widespread use of cell transplantation. Thanks to cell xenotransplantation techniques, xenocells from other species can be used in human recipients to treat a variety of ailments. For instance, advances in islet isolation and preservation have led to pancreatic islet allotransplantation for type-1 diabetes becoming a reality. Clinical trials have shown promising scientific support demonstrating that islet transplantation has the potential to reverse diabetes.
Nanotechnology Promotes Transplant Survival
The use of nanotechnology for therapeutic purposes has been fundamental in delivering various drugs and vaccines in the past few decades.
However, nanomedicine is advancing with promising applications in cancer therapies, neuroscience, and transplantation. In transplantation, nanotechnology modulates alloimmune responses by treating allografts pre- and post-transplantation to prevent graft injuries and induce donor-specific tolerance.
Nanotechnology-based encapsulation systems support the engraftment of pancreatic islets in animal models and protect the cells from immune attacks. Nanotechnology-based drug delivery strategies can increase the potential of a drug and allow targeted and controlled drug delivery.
Immunological Barriers to Xenotransplantation
Xenotransplantation has proved to be daunting due to the high risks of organ rejection, which can occur in different stages, namely, hyperacute rejection, acute vascular rejection, cellular rejection, and chronic rejection.
Hyperacute Rejection: When the graft of the transplanted organ destroys within minutes to hours, the process is known as hyperacute rejection (HAR). The rejection is performed by antibodies that pre-exist in the recipient’s body. The binding of antibodies to the xenograft stimulates the activation of complement proteins, which further leads to the destruction of graft vasculature. HAR can be prevented by depleting the level of antibodies or inhibiting complement activation in the recipient.
- Acute Humoral Xenograft Rejection (AHXR)
Acute humoral xenograft rejection or delayed xenograft rejection is a phenomenon caused by humoral and cellar immune responses. This occurs when a recovered level of antibody results in graft failure. Massive interstitial hemorrhage, thrombosis, necrosis, loss of tubules, etc., are classic features of AHXR.
- Cellular Xenograft Rejection
Cellular xenograft rejection can be mediated by innate or adaptive immune responses, and it may occur days to weeks after transplantation. This type of rejection in xenografts is expected to be stronger than seen in allografts and overcoming the barriers will require sustained concentrations of immunosuppressive drugs.
Besides these kinds of rejection, the risk of transmission of infectious diseases is another problem for the recipient of organ transplants.
Other Potential Animal Donors
Chimpanzees were considered to be the potential organ source for xenotransplantation to humans as they are closest relatives to humans. The organ size and blood type of chimpanzees are similar to that of humans. However, chimpanzees are listed as endangered species, so the medical community resorted to other animals for conducting xenotransplantation to humans.
Although baboons are readily available, they are impractical as potential donors due to their small body size and infrequent blood group, long gestation period, increased risk of disease transmission, and a small number of offspring.
Hence, pigs are considered the best candidates for organ donation due to reduced risk of disease transmission, short gestation periods, large litters, and ease of breed making. In 2020, the Federal Bureau of Drug Administration approved the genetic modification of pigs for the use of their organs in humans.
Further advances in recombinant genetic engineering technologies could extend the application of xenotransplantation to treat a variety of disorders in humans.
Website: https://www.techsciresearch.com/



